Researchers from IOCB Prague are furthering the understanding of how medicines work and what it takes to develop their most effective variants. In one current study, they have focused on the disease caused by the protozoan parasite Trichomonas vaginalis, especially because of the recent appearance of strains that are resistant to conventional treatment. In an effort to find a new weak spot of this parasite, the research group led by Dr. Evžen Bouřa has succeeded in preparing a key enzyme complex – the proteasome. This has made it possible to gain knowledge that is indispensable for the development of new effective medicines. The research was carried out in collaboration with colleagues from the University of California San Diego. An article presenting the work has been published in the scientific journal Nature Communications.
The proteasome is a complex protease that works as a device for recycling old proteins in cells. It is a cylindrical enzyme complex composed of seven subunits arranged in four superposed circles. When old proteins enter this cylinder, they get broken down into small pieces later used by the cell to make new proteins. If it functions incorrectly, old proteins accumulate, leading to the cell’s demise. This is why the blocking of proteasome activity is used, for example, against certain types of malignant tumours.
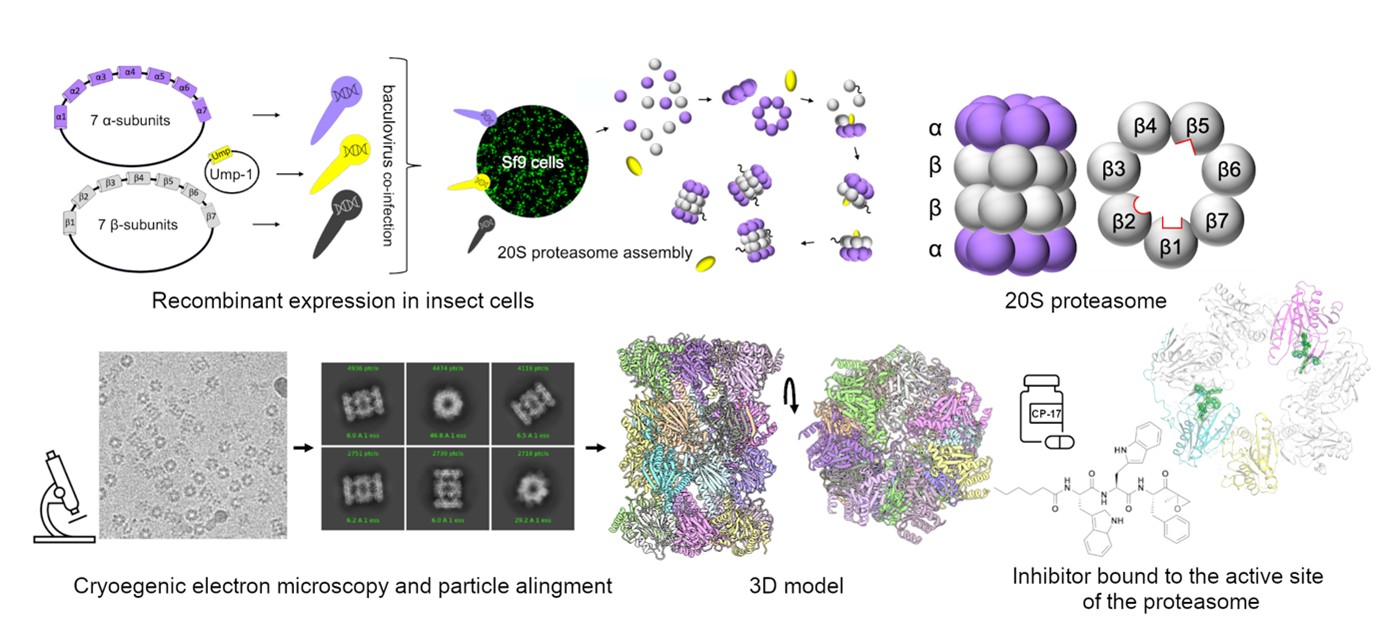
In the same way, it should also be possible to treat the widespread sexually transmitted infection trichomoniasis, which increases the risk of contracting HIV. The aim is therefore to identify substances that block the function of the proteasome inside Trichomonas vaginalis cells. However, the proteasome of this single-cell organism had never been satisfactorily isolated, so the research group from IOCB Prague prepared an artificial proteasome that is identical to the natural one. Curiously, they used insect cells to do so. This part of their research, on which the success of the whole project was hinged, proved the most complicated. That the team has prevailed in describing the previously unknown structure of the parasite’s proteasome is therefore of critical importance.
‘The proteasome looks the same in every animal or protozoan, but the details of this cylindrical structure differ. It is these details that we need to know in order to create a substance that is toxic to the parasite but not harmful to the human body. That is a crucial precondition for drug development. Our greatest success is that we have been able to prepare a recombinant, artificial proteasome that can provide us with the necessary information,’ explains Evžen Bouřa.
The researchers used advanced electron microscopy, allowing them to see almost every single atom. Armed with this capability, they tested the effects of two potential proteasome-inhibiting substances. They saw how they bind to the active site of the proteasome and under what circumstances the proteasome stops working, which is fatal to the cell of the parasite.
The research was carried out within the National Institute of Virology and Bacteriology (LX22NPO5103), funded by the EXCELES program.
Original article
- Silhan, J.; Fajtova, P.; Bartosova, J.; Hurysz, B. M.; Almaliti, J.; Miyamoto, Y.; Eckmann, L.; Gerwick, W. H.; O’Donoghue, A. J.; Boura, E. Structural elucidation of recombinant Trichomonas vaginalis 20S proteasome bound to covalent inhibitors. Nat. Commun. 2024, 15, 8621. https://doi.org/10.1038/s41467-024-53022-w