Tuberculosis, caused by Mycobacterium tuberculosis (Mtb), is one of the world's deadliest infectious diseases and remains a global health threat, killing over a million people each year. Urgent efforts in research and treatment are crucial to combat this ancient yet persistent pathogen.
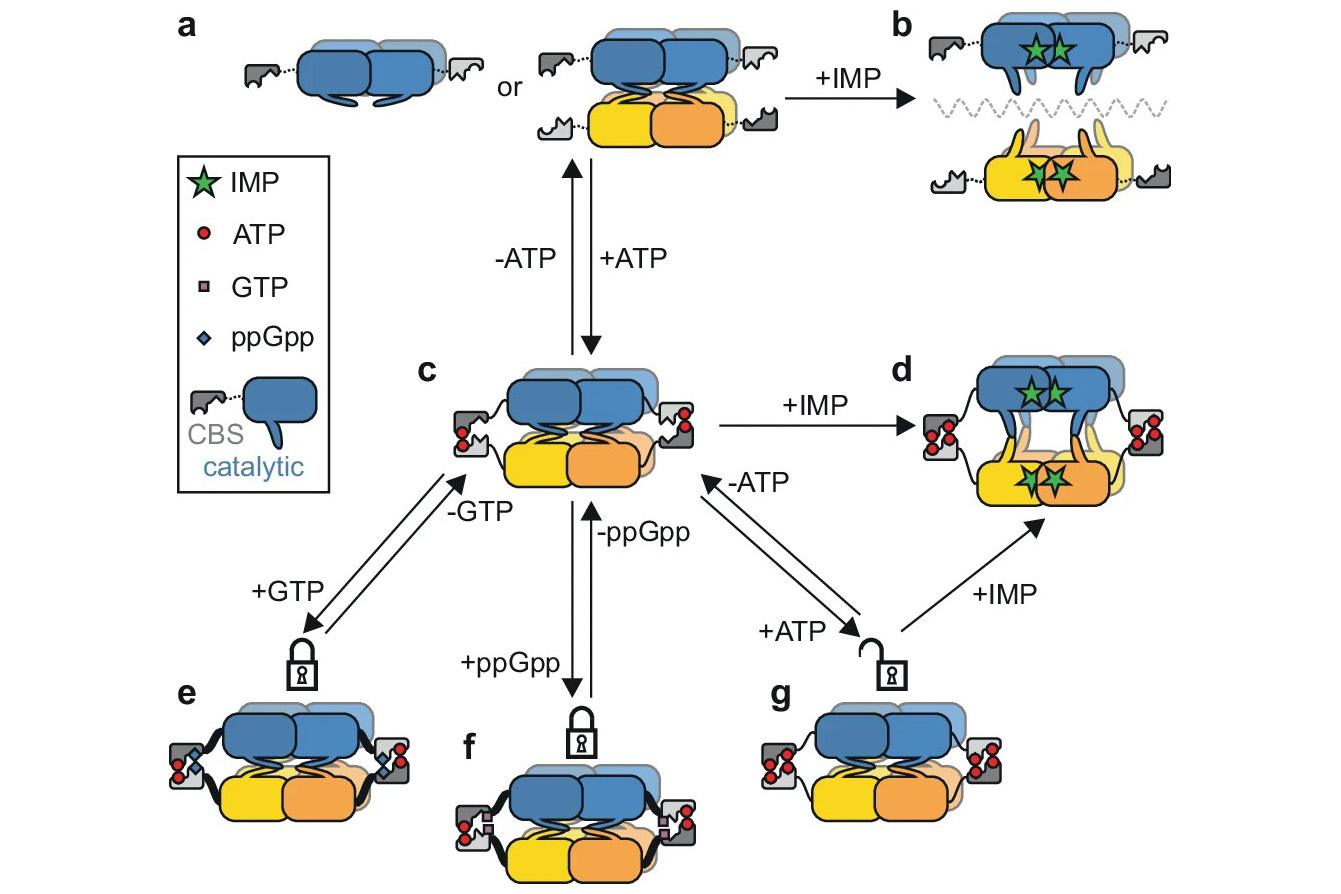
Researchers led by Tomáš Kouba and Iva Pichová from IOCB Prague have shed light on the detailed workings of a crucial enzyme called IMPDH (inosine 5′-monophosphate dehydrogenase), which plays a key role in purine metabolism, creating essential molecular building blocks in Mtb. They discovered that the IMPDH enzyme can be inhibited by a combination of ATP and either GTP or (p)ppGpp nucleotides. By using a series of cryogenic electron microscopy structures, the scientists meticulously mapped out how these molecules interact with the enzyme in different states, causing it to change shape and block its activity.
This discovery opens the door to designing and developing new antibacterial drugs that can target and inhibit IMPDH, potentially leading to effective treatments for Mtb infections.
The detailed mechanisms and results of this research were published in the journal Nature Communications, with Ondřej Bulvas as the first author.
This work was supported by the National Institute of Virology and Bacteriology (EXCELES, LX22NPO5103), funded by the EU, Next Generation EU program.
Original study
- Bulvas, O.; Knejzlík, Z.; Sýs, J.; Filimoněnko, A.; Čížková, M.; Clarová, K.; Rejman, D.; Kouba, T.; Pichová, I. Deciphering the allosteric regulation of mycobacterial inosine-5′-monophosphate dehydrogenase. Nat. Commun. 2024, 15, 6673. https://doi.org/10.1038/s41467-024-50933-6






