New prodrug activation method may enable faster and more effective treatment of severe diseases

Researchers from IOCB Prague have succeeded in preparing compounds capable of activating prodrugs at predetermined locations in the body, enhancing their effectiveness and expediting their action. This will make it possible, for example, to more precisely target malignant tumours, which might greatly advance the potential of anti-cancer therapies. Compounds referred to as new-generation prodrug activators have been developed in the laboratory of Dr. Milan Vrábel, and two research articles on the matter have been published in the scientific journal Angewandte Chemie.
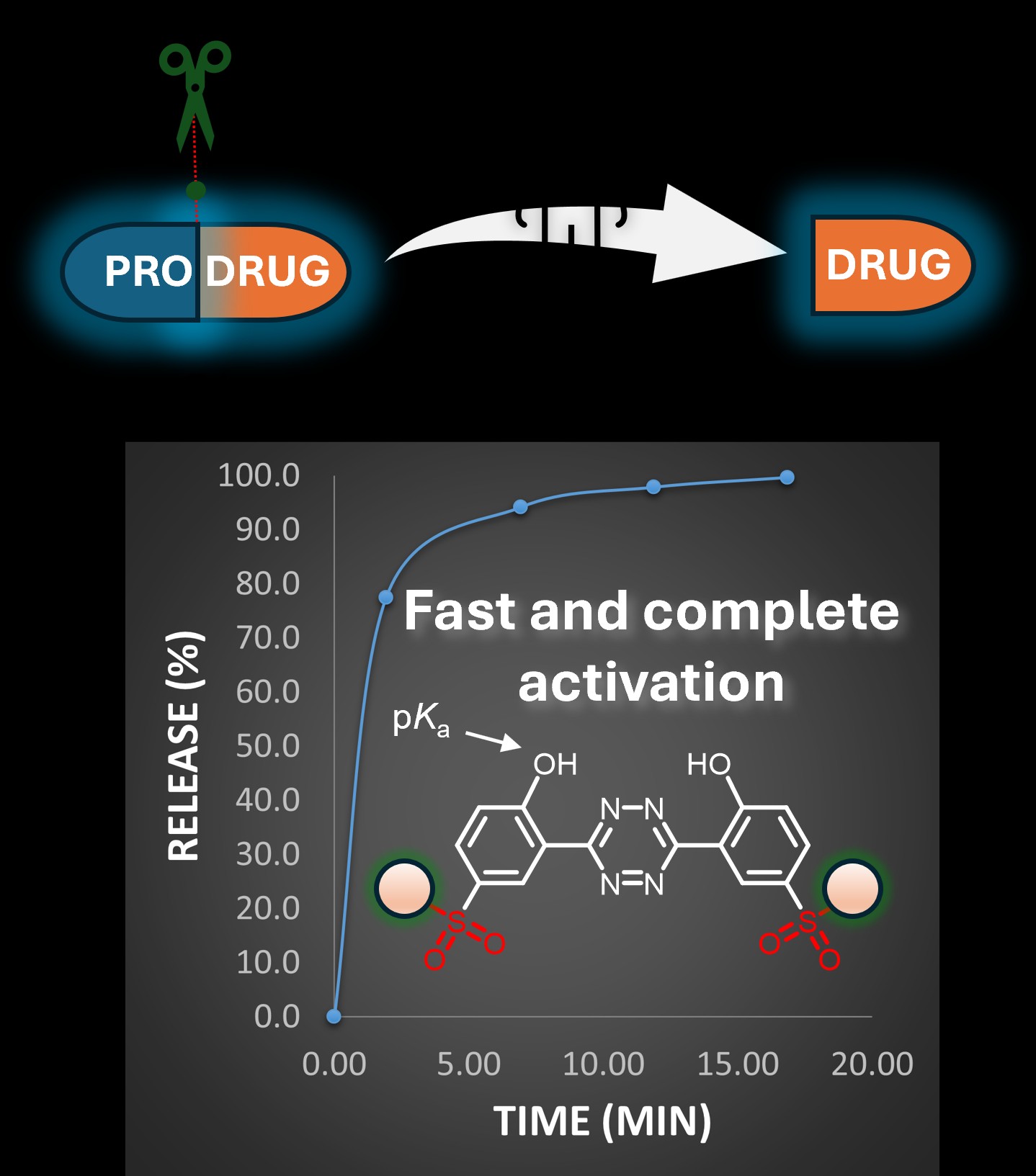
One objective of modern medicine is to utilize substances known as prodrugs, which are medicinal compounds that can be activated by being metabolically converted into drugs in specific parts of the body, for example near or inside cancerous tumours. It is not an easy process, because prodrugs tend to initiate their function in all sorts of places in the body. This is where compounds known as activators come into play. With their application, a specific chemical reaction is used to "unpack" the prodrug in the right location. However, the current generation of activators is slow and ineffective. Molecules of an active substance are usually released only after several hours, and the body can typically utilize only about 60% of the administered dose. Therefore, a research team at IOCB Prague, in collaboration with colleagues from the Technical University of Vienna, is working towards markedly enhancing the properties of prodrug activators.
"Our latest generation of activators allows for the complete release of the drug in a very short time of about half an hour," describes Milan Vrábel, adding: "We have proved the greater release speed and pharmacological efficacy in several exemplary in vitro cellular experiments."
The use of prodrug activators is being explored not only by researchers in basic science, but also by the pharmaceutical industry, and the first phase of clinical trials have already been completed. Although these are early, imperfect versions of prodrug activators, there is already evidence that this concept works in the human body. If, thanks to the research carried out at IOCB Prague, the properties of these molecules are successfully improved, more effective treatments for various diseases, including cancer, will be within reach.
Among other sources of funding, the research has been carried out thanks to the support of the project National Institute for Metabolic and Cardiovascular Disease Research (CarDia), funded by the EU's EXCELES program (LX22NPO5104), and within the framework of the NETPHARM project, also co-financed from European funds. In addition, support also came from national sources: the Czech Science Foundation and the Austrian Science Fund (FWF).
Original articles
- Rahm, M.; Keppel, P.; Dzijak, R.; Dračínský, M.; Šlachtová, V.; Bellová, S.; Reyes-Gutiérrez, P. E.; Štěpánová, S.; Raffler, J. E.; Tloušťová, E.; Mertlíková-Kaiserová, H.; Mikula, H.; Vrabel, M. Sulfonated Hydroxyaryl-Tetrazines with Increased pKa for Accelerated Bioorthogonal Click-to-Release Reactions in Cells. Angew. Chem. Int. Ed. 2024, e202411713. https://doi.org/10.1002/anie.202411713
- Wilkovitsch, M.; Kuba, W.; Keppel, P.; Sohr, B.; Löffler, A.; Kronister, S.; Fernandez del Castillo, A.; Goldeck, M.; Dzijak, R.; Rahm, M.; Vrabel, M.; Svatunek, D.; Carlson, J.; Mikula, H. Transforming Aryl-Tetrazines into Bioorthogonal Scissors for Systematic Cleavage of trans-Cyclooctenes. Angew. Chem. Int. Ed. 2024, e202411707. https://doi.org/10.1002/anie.202411707






