Birch reduction, a pivotal chemical reaction pioneered in the 1940s, holds significant importance in both organic and medicinal chemistry realms. This reaction is based on the selective protonation of aromatic hydrocarbons using alkali metals in liquid ammonia as reducing agents. While highly efficient, this method poses challenges for organic chemists due to the need for refrigerated liquid ammonia, which in addition emits volatile, malodorous, and irritating vapors.
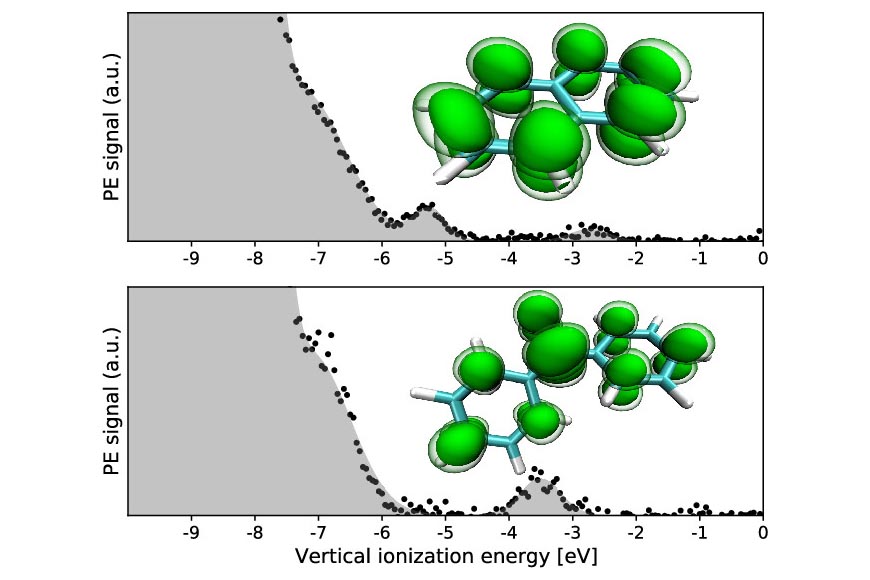
In pursuit of more accessible alternatives, scientists have sought room-temperature solvents conducive to Birch reduction. A team led by Christian Schewe from IOCB Prague (Pavel Jungwirth Group) and the J. Heyrovský Institute of Physical Chemistry and Stephen E. Bradforth from the University of Southern California in collaboration with researchers from the Fritz Haber Institute and Helmholtz Zentrum Berlin successfully applied photoelectron spectroscopy and quantum chemistry calculations and characterized radical anions of naphthalene and benzophenone as key Birch intermediates in a room temperature solvent – tetrahydrofuran.

Significantly, they demonstrated that the stability of these intermediates depends primarily on the dielectric constant of the liquid environment. This finding paves the way for designing optimal solvent mixtures conducive to enhancing the Birch reduction process, thus addressing the need for safer and more user-friendly alternatives to liquid ammonia.
Read the paper: Nemirovich, T.; Young, B.; Brezina, K.; Mason, P. E.; Seidel, R.; Stemer, D.; Winter, B.; Jungwirth, P.; Bradforth, S. E.; Schewe, H. C. Stability and Reactivity of Aromatic Radical Anions in Solution with Relevance to Birch Reduction. J. Am. Chem. Soc. 2024. https://doi.org/10.1021/jacs.3c11655