An elaborate chemical design of macrocyclic nanocarbons with helicene building blocks can lead to novel molecular architectures exhibiting remarkable (chir)optical properties, self-assembly, charge/spin transport, induced ring current or a fascinating Möbius topology.
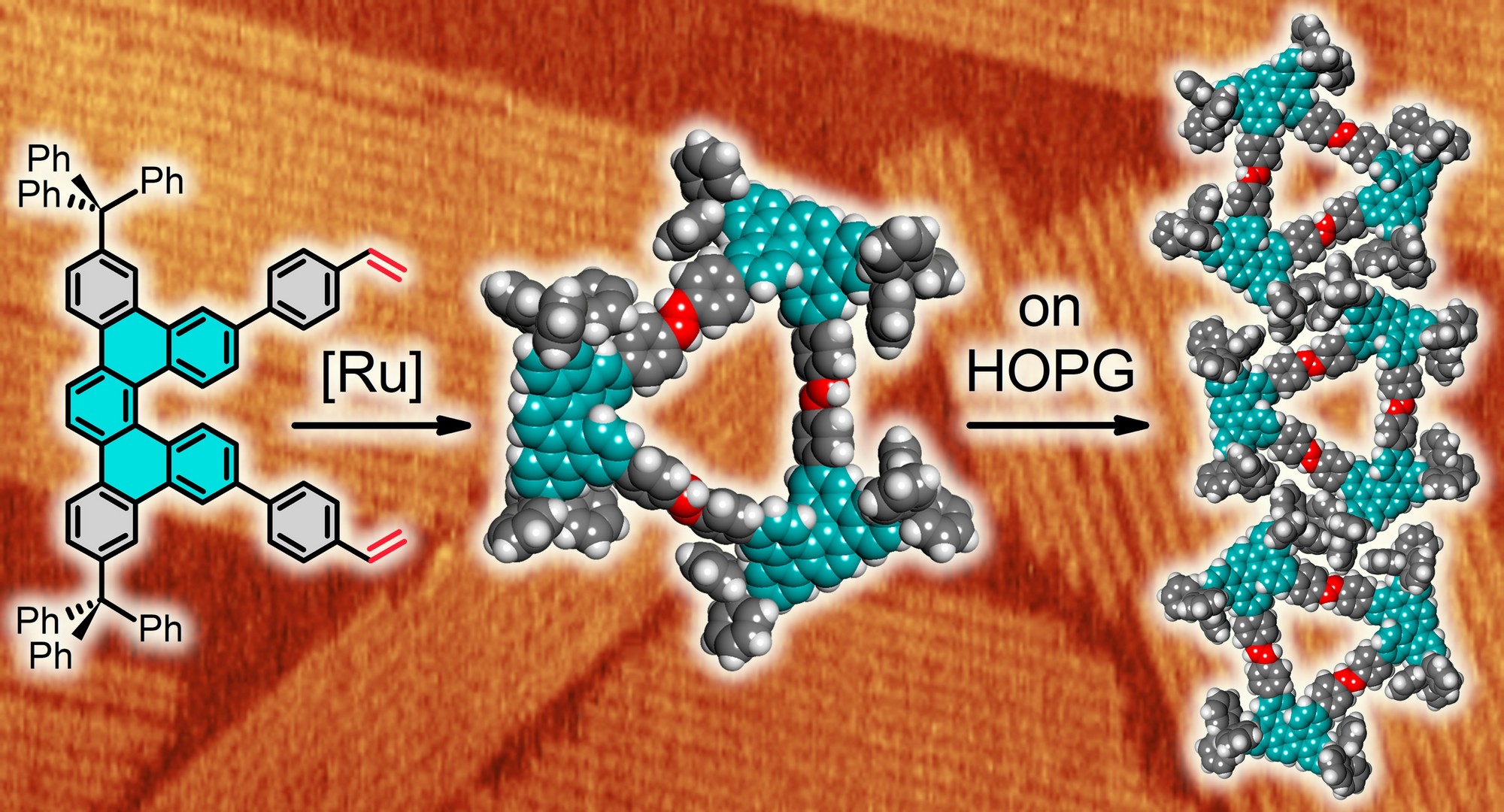
The scientists led by Ivo Starý and Irena G. Stará with the key contribution of Václav Houska from IOCB Prague in collaboration with Charles University, Institute of Physics, and the team from CTU in Prague led by Bohuslav Rezek designed, synthesized and studied helically chiral macrocycles bearing three or four angular dibenzo[5]helicene units as corners connected by aromatic linear linkers. The calculations revealed that the π-conjugation along the large, twisted trimer is rather disrupted, stabilizing this formally antiaromatic macrocycle by converting it to a non-aromatic system.
Atomic force microscopy study showed that the macrocyclic trimers self-assemble on graphite surface into long 1D stripes to form stripe-shaped domains stabilized through interactions between trityl groups attached to dibenzo[5]helicene units, thus working as a molecular Velcro. The results significantly expand the very limited knowledge of helically chiral π-electron macrocycles and show that such macrocycles can efficiently self-assemble into well-ordered 2D molecular crystals.
The results were published in Nanoscale with Václav Houska as the first author: Houska V.; Ukraintsev, E.; Vacek, J.; Rybáček, J.; Bednárová, L.; Pohl, R.; Stará. I. G.; Rezek, B.; Starý, I. Helicene-based π-conjugated macrocycles: their synthesis, properties, chirality and self-assembly into molecular stripes on a graphite surface. Nanoscale 2023. https://doi.org/10.1039/D2NR04209F






