Tomáš Slanina of IOCB Prague was one of two experimental chemists collaborating with a team of theoreticians headed by Joakim Bergman of the pharmaceutical company AstraZeneca and Henrik Ottosson of Uppsala University on a study of benzene reactivity in the excited state. The results of the joint effort were recently published in the Journal of American Chemical Society.
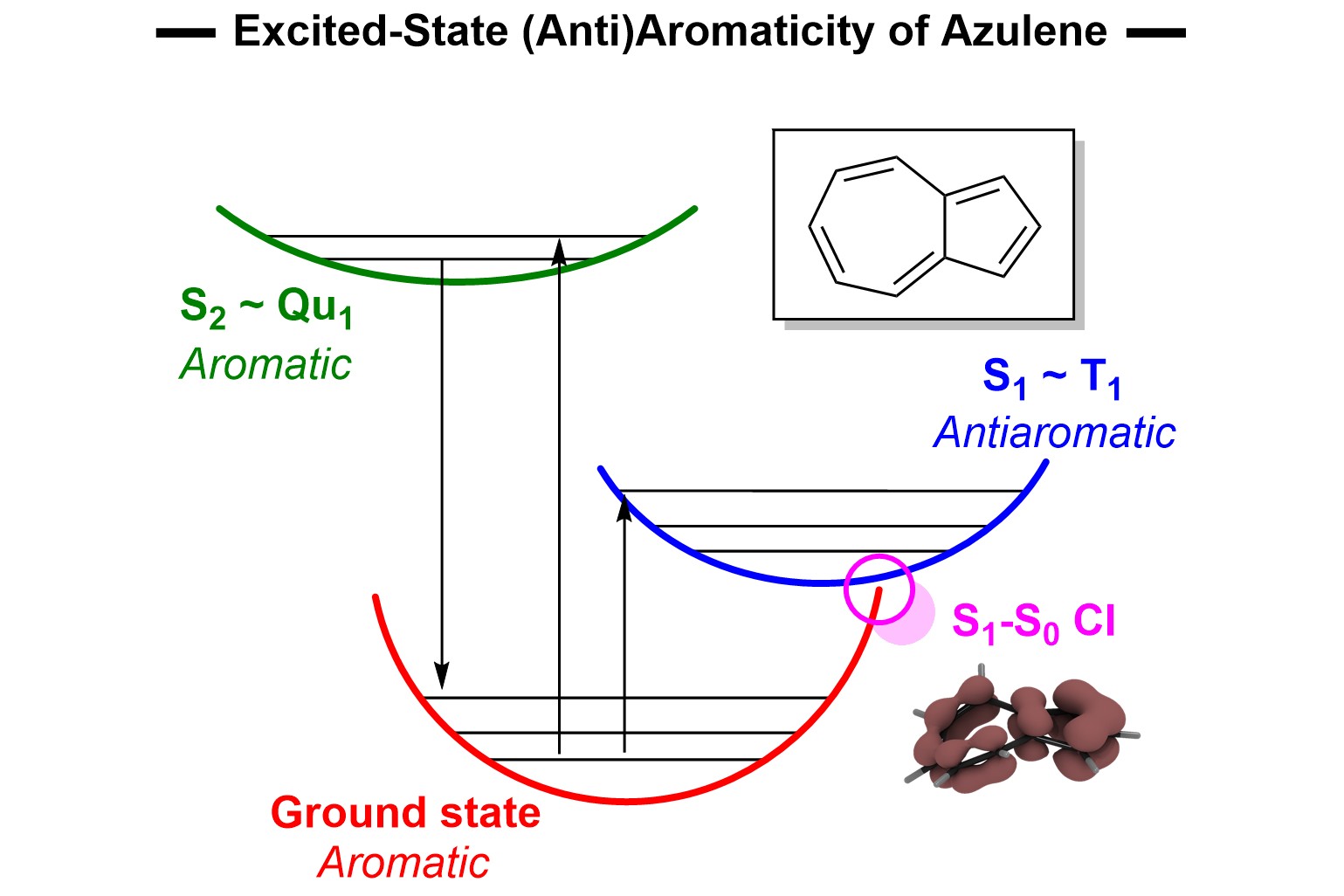
Unlike benzene in the ground state, which is aromatic and quite stable, its excited states are antiaromatic. As such, they are relatively unstable and known to undergo rearrangement combined with nucleophilic solvent addition, yielding strained and rigid bicyclo[3.1.0]hexenyl derivatives. The exact mechanism of this photochemical transformation, however, was not previously known.
Through calculations and experimental work, including isotopic labeling beginning with benzene-d6 and subsequent 2H-NMR, the researchers confirmed that the driving force of the reaction is the relief of antiaromaticity with the mechanism containing benzvalene as an intermediate. Furthermore, they determined that benzvalene is sensitized by excited benzene, leading to its rearomatization and reducing the quantum yield by a large margin.
Because the reaction results in the formation of three stereogenic centers, and the rearrangement intermediates undergo multiple complicated equilibria, considerable effort was made to shed light on the possibility of controlling the reaction’s stereochemistry. The researchers deciphered several substitution and condition patterns that lead to vastly different isomers as their final product, thus broadening the potential synthetic applicability of this reaction.
By partially untangling the decades-old enigma of benzene photochemistry, the study presents both a significant step in benzene reactivity theory and a potential synthetic tool for building a well-defined small rigid scaffold with potential for pharmaceutical applications, among others.
Original paper: Slanina T., Ayub R., Toldo J., Sundell J., Rabten W., Nicaso M., Alabugin I., Fdez. Galván I., Gupta A. K., Lindh R., Orthaber A., Lewis R. J., Grönberg G., Bergman J., Ottosson H.: Impact of Excited-State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]Hexenes. J. Am. Chem. Soc., May 26, 2020. https://doi.org/10.1021/jacs.9b13769