New molecules from IOCB Prague decrease appetite and protect the brain against Alzheimer’s-type diseases

Scientists on the team of Dr Lenka Maletínská have developed a promising new compound derived from one of the peptides naturally occurring in the brain. Its application may contribute to the addressing of two major health challenges of the modern days: obesity and Alzheimer's disease. The neuropeptide CART is primarily associated with the regulation of food intake. Its modified version, created at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, shows better stability and is more effective. It suppresses appetite and protects the brain by reducing the pathogenicity of the tau protein, which is associated with the dreaded Alzheimer's disease. The results of the research have been published in the European Journal of Pharmacology.
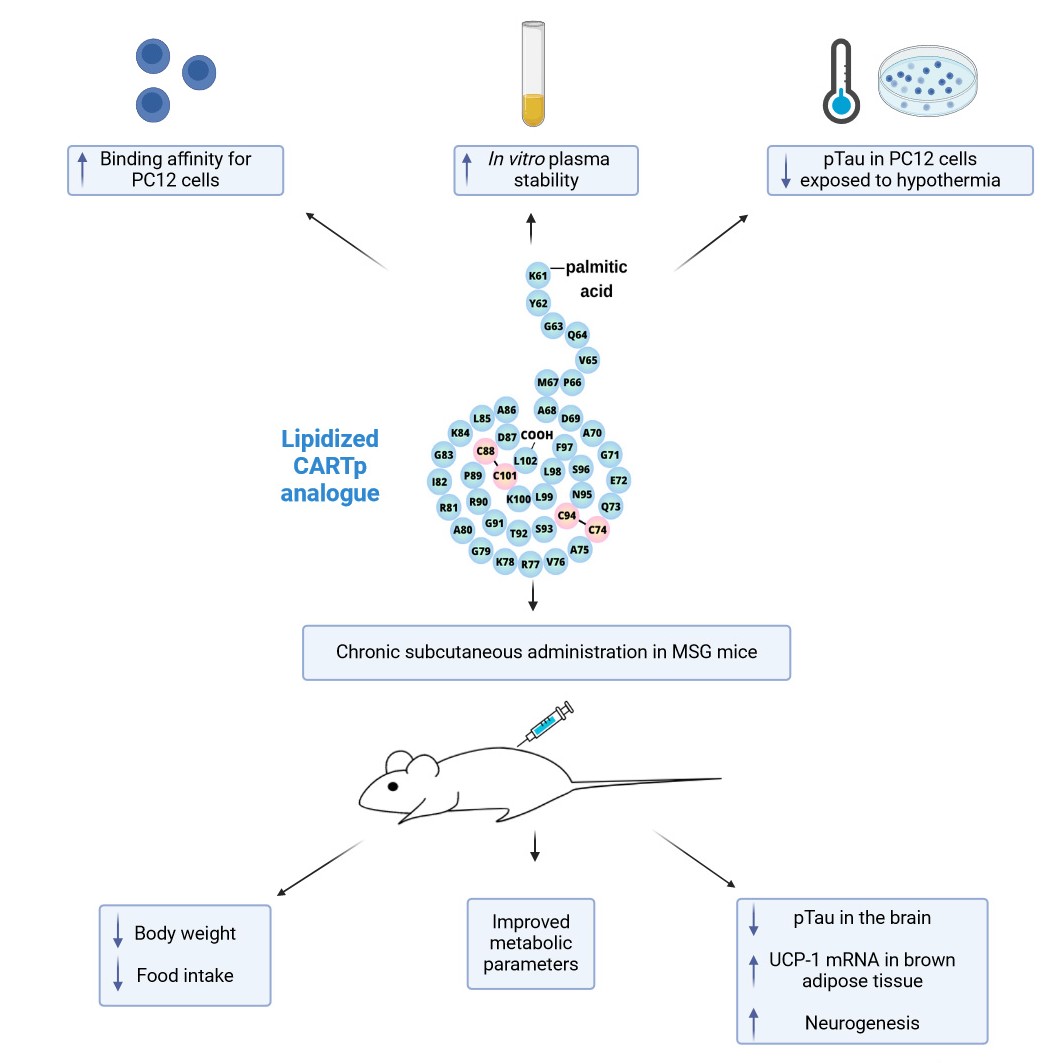
The new compound has successfully passed tests in both cell culture and animal models. Mice that were obese and prone to diabetes lost weight after its administration, and it turned out that they also exhibited reduced pathology of the dangerous tau protein, one of the main symptoms of Alzheimer's disease, in their brains. The modified molecule is effective in the body thanks to a process called lipidization. This means that the scientists have bound various fatty acids to the natural peptide CART and found that the modified peptide is able to cross the blood-brain barrier. This is a crucial precondition for the drug to work properly in the brain.
"We have found that when a lipidized analogue of the CART peptide is applied subcutaneously, it then passes into the brain, where it acts by suppressing appetite and, if administered for a long time, also has a neuroprotective effect. It could therefore work in the treatment or prevention of neurodegenerative diseases," explains the first author of the study Vilém Charvát.
The CART (cocaine- and amphetamine-regulated transcript) peptide itself was discovered in 1998 by the Danish pharmaceutical company Novo Nordisk. It has emerged that the peptide is abundant in the hypothalamus and that it has a relatively complex structure containing three disulfide bridges. However, what receptor in the body it binds to remains to be determined. Efforts to find out have so far not met with success. This is also the main goal in the sights of the principal author of the current study conducted at IOCB Prague, Dr Andrea Pačesová, who states: “We have a potentially successful anti-obesity drug in hands that also appears to reduce the risk of Alzheimer's disease. To develop this potential to its fullest, we need to know why the substance works the way it does. If, however, we want to describe the mechanism of its action, we must first decipher how the peptide gets to the brain. We know that it works. What is left to do is to identify the right receptors.”
Research into peptides potentially useful in the development of anti-obesity drugs at IOCB Prague is led by Dr Lenka Maletínská. A few years ago, she achieved the conclusion of a licensing agreement for a promising substance with the pharmaceutical company Novo Nordisk. She herself has long assumed that anorexigenic (appetite-lowering) peptides might also be used for the prevention and treatment of neurodegenerative diseases. “We already know, contrary to what was originally assumed, that new neurons are formed even in adulthood. This regenerative process is supported by several anorexigenic peptides, which can thus help repair damaged brain tissue,” says Dr Maletínská, adding that: “If Alzheimer's disease can be diagnosed early on, in a phase called mild cognitive impairment, the chances of it being cured appear to be quite high.”
Original article
- Charvát, V.; Strnadová, A.; Myšková, A.; Sýkora, D.; Blechová, M.; Železná, B.; Kuneš, J.; Maletínská, L.; Pačesová, A. Lipidized analogues of the anorexigenic CART (cocaine – and amphetamine-regulated transcript) neuropeptide show anorexigenic and neuroprotective potential in mouse model of monosodium-glutamate induced obesity. Eur. J. Pharmacol. 2024, 980. https://doi.org/10.1016/j.ejphar.2024.176864