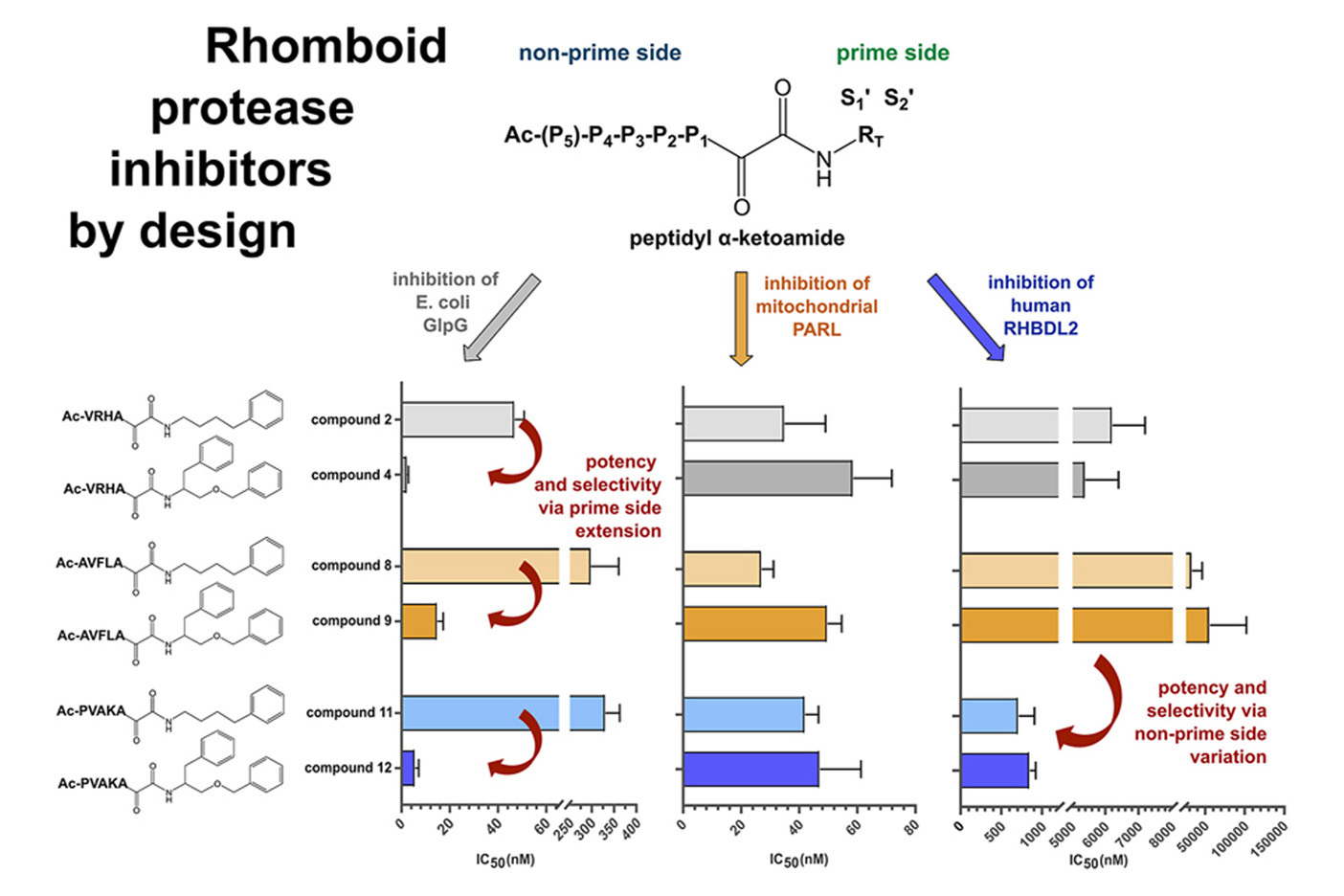
An interdisciplinary and collaborative research team led by Kvido Stříšovský from IOCB Prague aimed to improve the selectivity of ketoamide inhibitors for different rhomboid proteases and enhance their efficacy through structural modifications. Rhomboids are widespread intramembrane serine proteases fulfilling manifold physiological functions within all domains of life, and rhomboid proteases have repeatedly been associated with various diseases, becoming attractive novel pharmacological targets.
Key findings of the team’s recent study in the Eur. J. Med. Chem. show that branched substituents significantly enhance the selectivity and efficacy of ketoamide inhibitors for specific rhomboid proteases. Structural analysis revealed that these modifications allow the inhibitors to better fit the active sites of target proteases. However, the research also highlights the challenge of lacking suitable in vitro models for developing effective inhibitors for eukaryotic rhomboid enzymes.
The study opens several pathways for further research and development. These include developing and validating in vivo models, exploring therapeutic applications for diseases associated with rhomboid proteases, and conducting broader chemical exploration to identify other effective modifications.
Read the paper: Bach, K.; Dohnálek, J.; Škerlová, J.; Kuzmík, J.; Poláchová, E.; Stanchev, S.; Majer, P.; Fanfrlík, J.; Pecina, A.; Řezáč, J.; Lepšík, M.; Borshchevskiy, V.; Polovinkin, V.; & Strisovsky, K. Extensive targeting of chemical space at the prime side of ketoamide inhibitors of rhomboid proteases by branched substituents empowers their selectivity and potency. Eur. J. Med. Chem. 2024, 275, 116606. https://doi.org/10.1016/j.ejmech.2024.116606