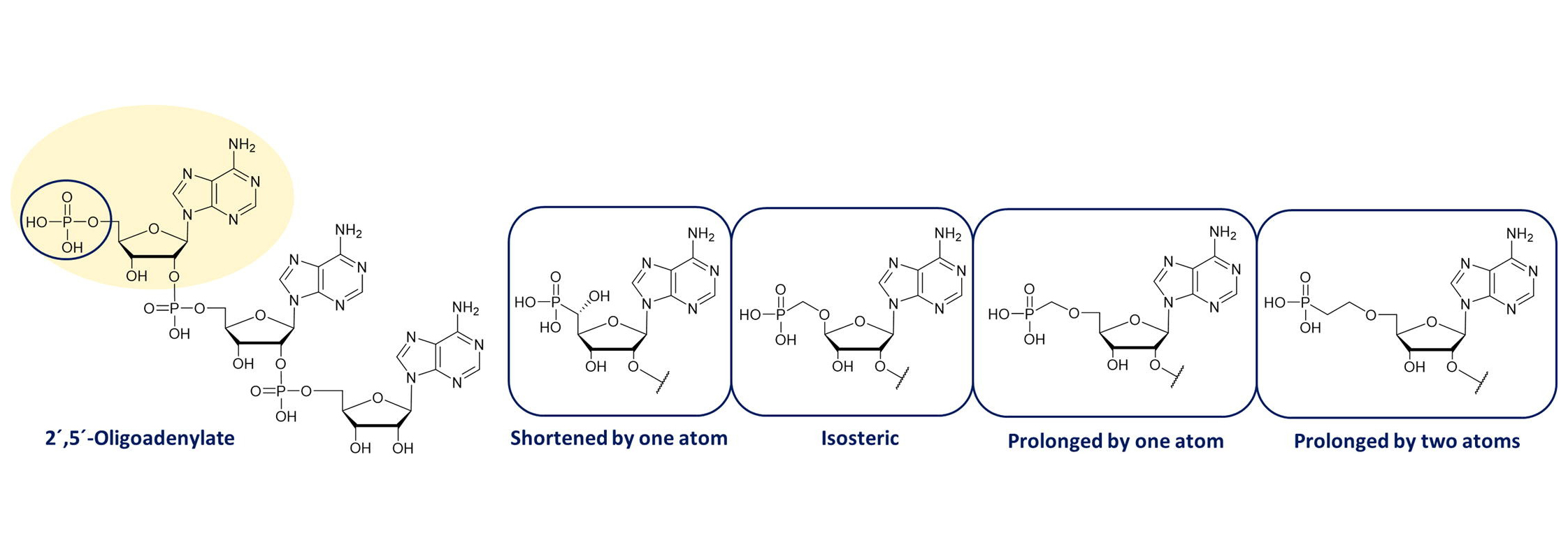
The oligoadenylate synthetase-ribonuclease L pathway (OAS-RNase L) is a major player in the interferon-induced antiviral defense mechanism of cells. Upon sensing viral dsRNA, 5´-phosphorylated 2´,5´-oligoadenylates are synthesized, and subsequently activate latent RNase L. The activation of the OAS-RNase L pathway leads to the inhibition of the synthesis of viral proteins and induces apoptosis of infected cells, and therefore directly restricts the replication of viruses.
The scientific team led by Ivan Rosenberg and Ondřej Páv from IOCB Prague determined the influence of the 5′-phosphate end of 2-5A on the activation of human RNase L. They developed synthetic routes leading to nine novel phosphonate monomers suitable for the solid-phase synthesis of phosphonate-modified 2-5A oligonucleotides. The scientists tested four sets of 2-5A oligonucleotides bearing shortened, isosteric, and prolonged phosphonate linkages. The understanding of the biological activity of synthetic RNase L activators can bring promising results in developing new antiviral drugs.
Read the paper:
- Lášek, T.; Petrová, M.; Košiová, I.; Šimák, O.; Buděšínský, M.; Kozák, J.; Snášel, J.; Vavřina, Z.; Birkuš, G.; Rosenberg, I.; Páv, O. 5′-Phosphonate modified oligoadenylates as potent activators of human RNase L. Bioorg. Med. Chem. 2022, 56, 116632. https://doi.org/10.1016/j.bmc.2022.116632





