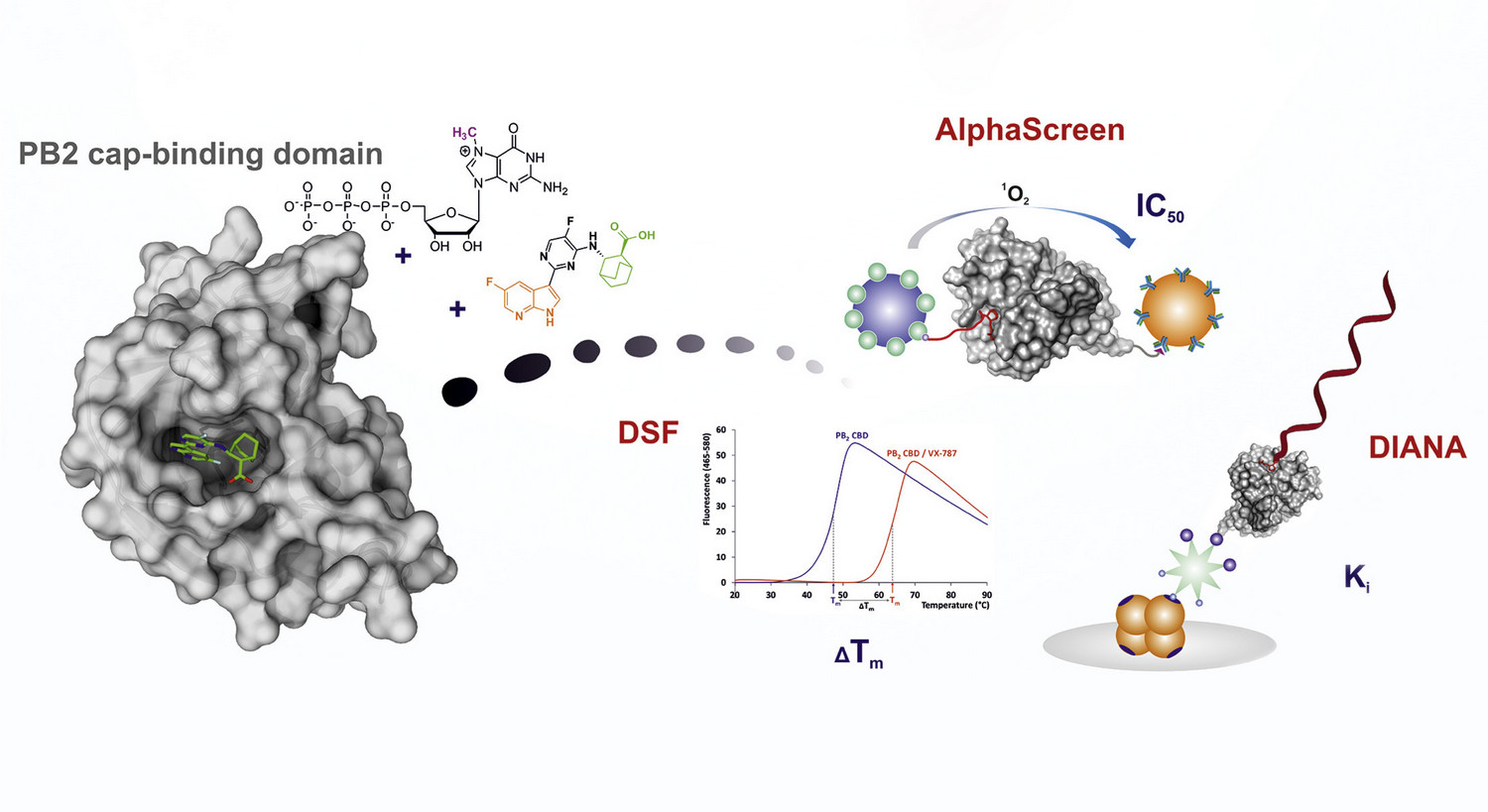
The interdisciplinary team led by Milan Kožíšek from Jan Konvalinka Group and Aleš Machara from Pavel Majer Group at IOCB Prague continues with their research of influenza polymerase inhibitors.
In their previous research, the team showed that particular flavonoids are potent inhibitors of the influenza polymerase PA subunit that features endonuclease activity. To find more efficient inhibitors, this time around the scientists synthesized luteolin derivatives modified at the C-7 and C-8 positions and determined their IC50 values using AlphaScreen technology.
The most potent inhibitors proved to be the C-8 luteolin derivatives, while the C‐7 hydroxyl moiety of luteolin led to a series of compounds with one‐order‐of‐magnitude‐lower inhibitory potencies.
The researchers succeeded in solving the X-ray crystal structure of the wild‐type PA‐N‐terminal domain in complex with orientin. In addition, they elucidated the structure of the domain’s I38T mutant which causes resistance against flu treatment with Xofluza (Baloxavir marboxil).
The results were published in the International Journal of Molecular Sciences with Róbert Reiberger and Kateřina Radilová as first authors.
Read the paper:
- Reiberger, R.; Radilová, K.; Kráľ, M.; Zima, V.; Majer, P.; Brynda, J.; Dračínský, M.; Konvalinka, J.; Kožíšek, M.; Machara, A. Synthesis and In Vitro Evaluation of C-7 and C-8 Luteolin Derivatives as Influenza Endonuclease Inhibitors. Int. J. Mol. Sci. 2021, 22, 7735. https://doi.org/10.3390/ijms22147735