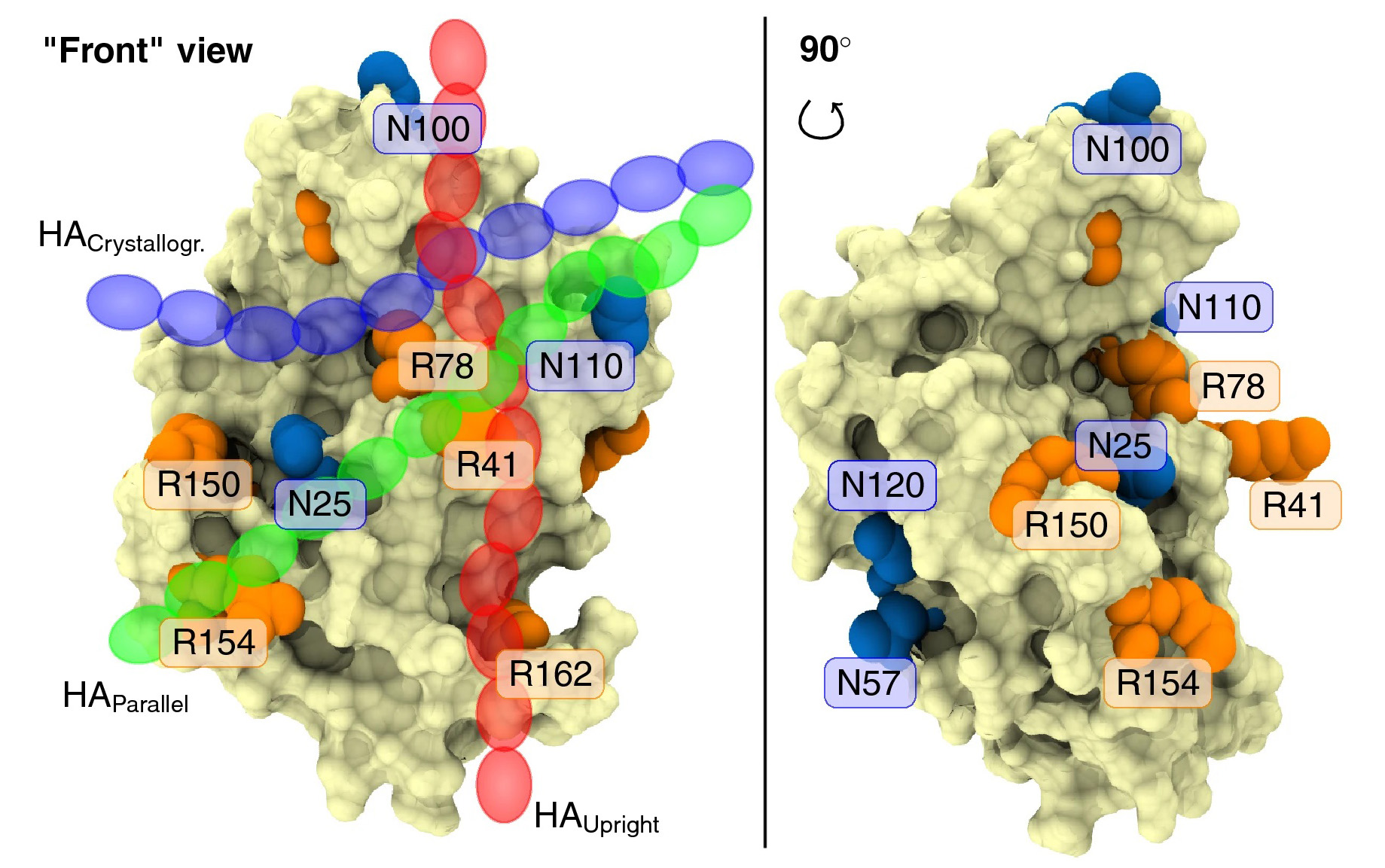
While DNA encodes protein structure, glycans can regulate their function. For example, N-glycosylation can modulate the ability of the cell surface receptor CD44 to bind its ligand, hyaluronan. However, despite its importance, the mechanism of this modulation remained unclear until now.
Based on atomistic simulations and NMR, researchers from IOCB Prague, University of Helsinki, and Institute of Molecular Genetics of the CAS, led by Hector Martinez-Seara, show in their recent paper in Scientific Reports that CD44 has multiple distinct binding sites for hyaluronan and that N-glycosylation modulates their respective roles. They found out that non-glycosylated CD44 favors the canonical sub-micromolar binding site, while glycosylated CD44 binds hyaluronan with an entirely different micromolar binding site.
These findings, for the first time, show how exactly glycosylation can alter receptor affinity. It shields specific regions of the host protein and thereby promotes the weaker binding modes.
The revealed mechanism emphasizes the importance of glycosylation in protein function. But it also poses a challenge for protein structure determination where glycosylation is usually neglected.
Read the paper:
- Vuorio, J.; Škerlová, J.; Fábry, M.; Veverka, V.; Vattulainen, I.; Řezáčová, P.; Martinez-Seara, H. N-Glycosylation can selectively block or foster different receptor–ligand binding modes. Sci. Rep. 2021, 11, 5239. https://doi.org/10.1038/s41598-021-84569-z






