The Interplay Between Antiaromaticity and Diradical Character in Diarenoindacenes and Diindenoarenes
Abstrakt
This talk will present our synthetic, structural, computational and materials studies of a class of carbon-rich semiconducting molecules based on the indenofluorene (IF) skeleton.1 Access to the fully conjugated, 20 π-electron, formally antiaromatic system is accomplished via a variety of intermediate diones. These molecules in turn can be assembled via well-known organic procedures (Suzuki cross-coupling, benzylic oxidation, Friedel-Crafts acylation/alkylation).1 Optimization of their synthesis now permits access to IF derivatives in multigram quantities. We have shown that thin films or single crystals of several different IF scaffolds can serve as an active layer in organic field effect transistors (OFETs).2 Current studies (Figure 1) are focused on varying the antiaromaticity of the indacene unit by systematic alteration of the outer benzene groups with other aromatic units3 as well as on increasing the diradical character of the framework by expansion of the quinoidal core.4
Keywords: antiaromaticity • diradical • organic semiconductor • NICS calculations
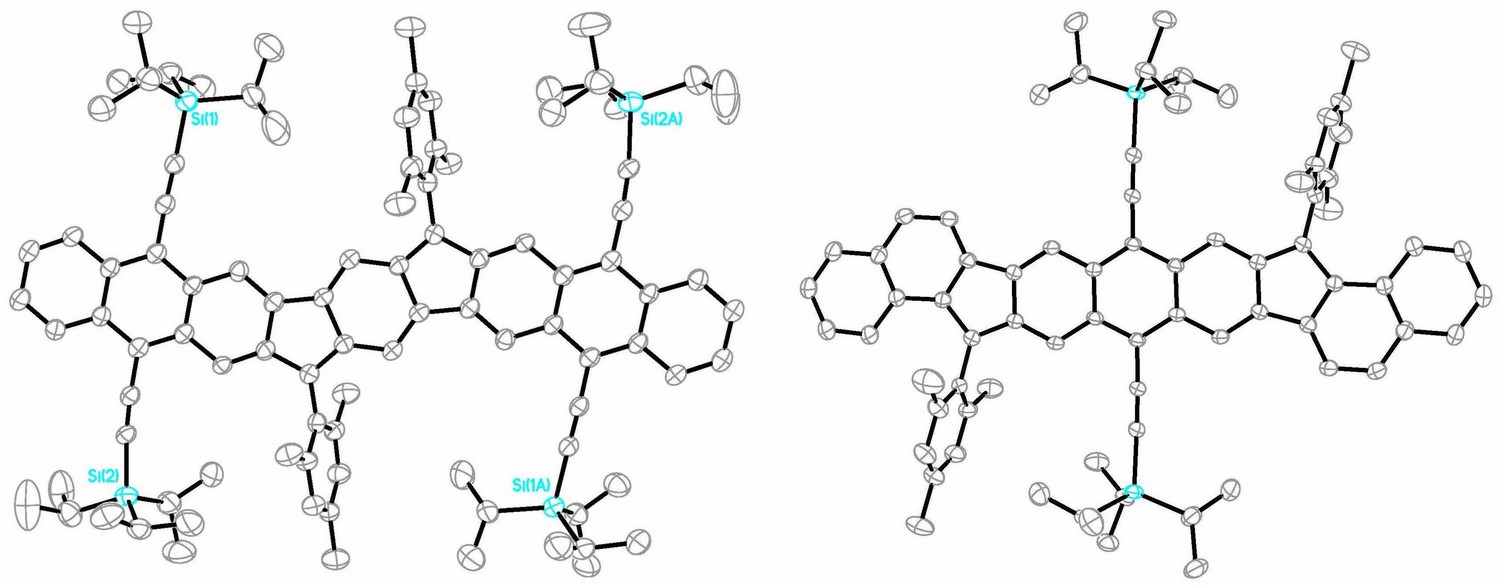
Zdroje
- (a) Frederickson, Rose & Haley, Acc. Chem. Res. 2017, 50, 977–987; (b) Dressler & Haley, J. Phys. Org. Chem. 2020, 33, e4114
- (a) Marshall et al., Chem. Sci. 2016, 7, 5547–5558; (b) Zeidell et al., Chem. Mater. 2019, 31, 6962–6970.
- (a) Frederickson, Zakharov & Haley, J. Am. Chem. Soc. 2016, 138, 16827–16838; (b) Frederickson et al., Synlett 2018, 29, 2562–2566. (c) Barker et al., Angew. Chem. Int. Ed. 2021, 60, 22385–22392.
- (a) Rudebusch et al., Nat. Chem. 2016, 8, 753–759; (b) Dressler et al., Nat. Chem. 2018, 10, 1134–1140; (c) Barker et al., J. Am. Chem. Soc. 2020, 142, 1548–1555; (d) Dressler et al., Chem 2020, 6, 1353–1368; (e) Hayashi, Barker et al., J. Am. Chem. Soc. 2020, 142, 20444–20455.



